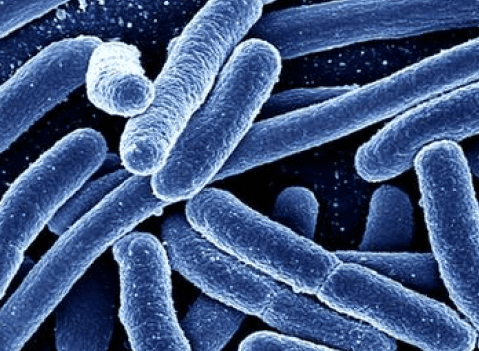
Hemolytic uremic syndrome was first described in 1955, but it was not known to be secondary to Escherichia coli (E. coli) infections until 1983. HUS is now recognized as a cause of acute kidney failure in infants and young children. Adolescents and adults are also susceptible, as are the elderly, who often have severe disease and are at significant risk of death from the disease.
If you have experience with this condition, you should know that Marler Clark provides hemolytic uremic syndrome lawyers so that you can get the compensation you deserve. We have worked with professional epidemiologists to provide clients with helpful resources regarding HUS as well.
If you live with hemolytic uremic syndrome and need help navigating your options, Marler Clark will help you get started.
Hemolytic Uremic Syndrome Resources
We have worked with epidemiologists to bring education about Hemolytic Uremic Syndrome to everyone.
Outbreaks and recalls
January 28, 2024
Common Causes of E. coli Outbreaks in Restaurants
E. coli O157:H7 bacteria and other pathogenic E. coli mostly live in the intestines of cattle, but E. coli bacteria have also been found in the intestines of chickens, deer...
January 20, 2024
The Litigated Dish: Can Foodborne Illness be Contagious?
This question, however, is quite broad because – which foodborne illness? There are several bacteria, viruses, and parasites that can cause foodborne illness. I thought I would put together a...
January 02, 2024
Technological Advancements That Help Fight E. coli
In 1993 after a large multi-state outbreak of E. coli O157 infections in the Western United States. To prevent future severe outbreaks an effective surveillance network called PulseNet was developed...
November 24, 2023
Common Myths and Misconceptions About E. coli
E. coli is often referred to as the best or most-studied free-living organism. More than 700 serotypes of E. coli have been identified. The “O” and “H” antigens on the...
Have Hemolytic Uremic Syndrome symptoms? Get a free consultation.
We’re here to answer all your questions, like what to ask a doctor, if you’re eligible for a lawsuit, and how to get the care you need.
Hemolytic Uremic Syndrome litigation at Marler Clark
The Marler Clark Hemolytic Uremic Syndrome attorneys are the only lawyers in the nation with a practice focused exclusively on plaintiff foodborne illness litigation.
Our expert Hemolytic Uremic Syndrome attorneys have represented victims of notable Hemolytic Uremic Syndrome outbreaks. Contact us today to learn more about our services.
See what our clients are saying
Marler Clark's food litigation attorneys have the most extensive experience representing victims of food poisoning outbreaks of any law firm in the United States.
Bill Marler and his team demonstrated a clear passion for their work and diligently ran to ground all of the details and nuances surrounding our family's case. The Marler Clark team managed our expectations extremely well, making sure that we were prepared at each step in the process and knew that there would be frustrating times along the way. On top of the impeccable professionalism, we formed friendships with Bill and Julie, and they introduced us to other clients who were going through similar experiences to our own, all of which was therapeutic and reminded us that we were not alone. And last but not least, we achieved success -- there is no substitute for subject matter expertise and years of experience! Thanks again Bill, Julie, and the entire Marler Clark team!
Bob & Emily S.
All of the people at Marler and Clark were very attentive to our needs and concerns. We would highly recommend their law firm for any legal advice regarding food safety. They are very transparent and will make contact with you in a timely fashion.
Amy G.
My wife and I can't thank Bill Marler and everyone at Marler Clark enough for how well they looked after us in our time of need. Bill visited us while our son was in the hospital and he, or his staff, were in contact with us every step of the way. Everyone at Marler Clark was caring and compassionate about our situation while working on our behalf. Even after our case was settled, Bill has checked in with us from time to time, wanting to know how our son was doing.